Abstract
Introduction
Burkitt lymphoma (BL) is an aggressive B-cell non-Hodgkin lymphoma with a hallmark translocation involving MYC and an immunoglobulin enhancer. It is most common in children and has three clinical variants: endemic, sporadic, and HIV-associated. The Epstein-Barr virus (EBV)-associated endemic subtype (eBL) is highly prevalent in equatorial Africa, where it is the most common pediatric cancer. Survival is poor among African children with eBL due to late stage at presentation and limitations in the ability to support intensive chemotherapeutic regimens necessary to induce durable remissions. Previous genomic studies of smaller BL cohorts revealed that eBL harbors mutation patterns similar but not identical to sporadic cases from high-resource countries. The BLGSP aims at conducting an integrative molecular characterization of a large comprehensive BL cohort including an unprecedented representation of endemic cases. The objective is to define molecular features that drive lymphomagenesis, which can be translated to new therapeutic strategies deployable worldwide.
Methods
Tumor biopsies and matched normal tissue have been collected from patients with both endemic and sporadic BL along with treatment, outcome, and other clinical information. Molecular characterization includes whole genome sequencing of tumor and constitutional DNA (80X and 40X, respectively), and strand-specific ribo-depleted sequencing of tumor RNA and microRNA. Six centroblast and 6 centrocyte samples flow-sorted from healthy donor tissue underwent RNA and miRNA sequencing as normal comparators for gene expression. These data enable the identification of somatic mutations, significantly mutated genes (SMGs) and non-coding loci with potential regulatory function; the quantification of human and EBV gene expression; and the characterization of miRNA-mediated regulation of transcripts. SMGs were supported by two or more of the following methods with a 10% false discovery rate: OncodriveFM, OncodriveFML, OncodriveCLUST, and MutSigCV. We also re-analyzed the genome data for 17 sporadic cases from the ICGC MALY-DE project (Nat. Genet. 2015; 47: 1316-1325).
Results
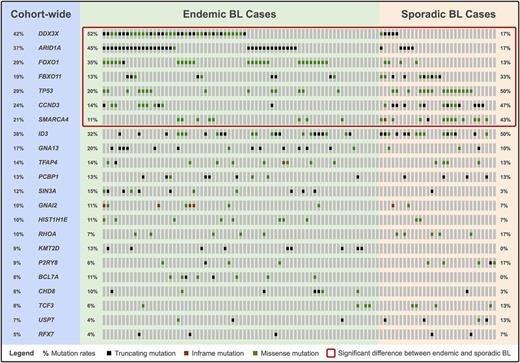
A total of 101 cases (71 endemic and 30 sporadic) were included after quality control. We describe novel mutation features affecting known BL genes. Notably, DDX3X is the target of focal deletions and splicing branch point mutations, and in-frame deletions recurrently affect a putative ubiquitination site (K272) in GNAI2 . Our meta-analysis reveals 22 SMGs, including many that have not yet been linked to the disease (see figure). We associate recurrent mutations in the following genes with BL for the first time: SIN3A, HIST1H1E, KMT2D, CHD8, USP7, and RFX7 . Several SMGs are differentially mutated between endemic and sporadic BL, suggesting distinct underlying biology (see highlighted genes in figure). For instance, ARID1A is preferentially mutated in endemic cases whereas SMARCA4 shows the opposite trend. Multiple non-coding loci are also significantly mutated: a PAX5 enhancer, BCL6 super-enhancers, the PVT1 non-coding locus, and the miR-142 gene, among others. Three mutation signatures are detected across all tumors: one is associated with defective DNA mismatch repair, another has been linked to polymerase η and AID activity, and the last signature has an unknown etiology. Gene expression analysis reveals high correlation between tumors (Pearson coefficient > 0.9). This is consistent with the absence of robust clusters according to unsupervised clustering of expression profiles.
Discussion
BLGSP is an ongoing international collaborative project aimed at providing a comprehensive molecular portrait of BL across all subtypes. We describe previously unreported mutation features affecting established BL genes and identify novel candidate coding and non-coding driver mutations, nominating compelling targets for therapy. For example, one of the novel SMGs, USP7, encodes a deubiquitinase that counteracts Mdm2-mediated ubiquitination and degradation of p53. We hypothesize that Mdm2 inhibitors might trigger synthetic lethality in USP7 -mutated, TP53 -wildtype tumors. In summary, this effort has identified candidate molecular targets for therapy that can potentially lead to more effective treatments that are less toxic than the current regimens.
BMG and DSG contributed equally. RDM and LMS contributed equally.
Casper: TempTime: Consultancy, Other: Travel, Accommodation, Expenses; GSK: Other: Travel, Accommodation, Expenses; Janssen: Consultancy, Research Funding; Up to Date: Patents & Royalties; Roche: Consultancy, Other: Travel, Accommodation, Expenses. Mullighan: Loxo Oncology: Research Funding; Amgen: Consultancy. Abramson: Abbvie: Consultancy; Genentech: Consultancy; LAM Therapeutics: Research Funding; Gilead: Consultancy; Kite Pharma: Consultancy; Seattle Genetics: Consultancy; Celgene: Consultancy; Novartis: Consultancy. Noy: Pharmacyclics LLC, an AbbVie Company: Honoraria, Other: Travel, Accommodation, Expenses, Research Funding, Speakers Bureau. Morin: Epizyme, Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal